Abstract
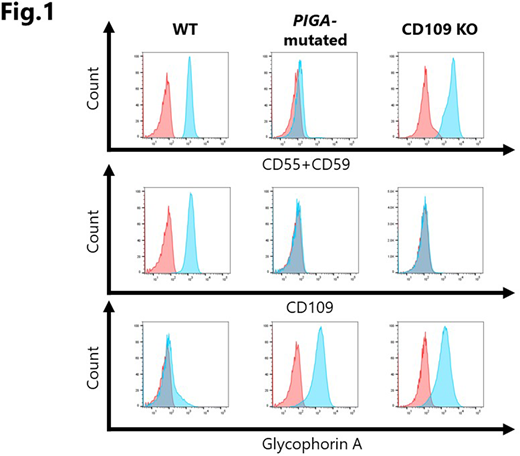
[Background] Glycosylphosphatidylinositol-anchored proteins (GPI-APs) on hematopoietic stem progenitor cells (HSPCs) may play an important role in the regulation of the HSPC commitment, given the fact that a lack of GPI-APs due to PIGA mutations allows HSPCs to preferentially undergo commitment into mature blood cells under immune pressure against HSPCs in patients with acquired aplastic anemia. CD109, one of the GPI-APs expressed by keratinocytes and HSPCs in humans, serves as a TGF-β co-receptor and is reported to inhibit TGF-β signaling in keratinocytes; however, the role of CD109 on HSPCs has not been clarified. TF-1 is one of a few myeloid leukemia cell lines that express CD109, the proliferation of which is dependent on GM-CSF. Since TF-1 undergoes erythroid differentiation in response to δ-5-aminolevulinic acid (δ-ALA), and its differentiation is reportedly inhibited by TGF-β, a lack of GPI-APs due to PIGA mutation and/or the knockout (KO) of CD109 may affect the differentiation of TF-1 cells. [Objectives/Methods] To gain insights into the role of GPI-APs on HSPCs, we established a PIGA-mutated TF-1 cell line by culturing TF-1 in the presence of α-toxin for several months, and a CD109 KO TF-1 cell line using a CRISPR-Cas 9 system. The erythroid differentiation of the cells was assessed by testing the expression of glycophorin A (GPA) on TF-1 cells using flow cytometry (FCM) and iron staining. We also determined the CD109 expression by HSPCs from healthy individuals and C57BL/6 mice using FCM and a quantitative PCR. [Results] Both GPI-AP-deficient TF-1 cells that had a PIGA mutation (7 nucleotide deletion at position 291-297 [TTGTCAC] in exon 2) and CD109 KO TF-1 cells showed slower proliferation than wild-type (WT) TF-1 cells. Similarly to TF-1 cells treated with δ-ALA, both mutant cells expressed GPA, exhibited erythroid morphology, and were positive for iron granules, suggesting that GPI-APs inhibited the erythroid differentiation of WT TF-1 cells that were cultured in RPMI1640 containing 10% fetal bovine serum (FBS), and that the GPI-AP that plays a key role in the inhibition of erythroid differentiation is CD109. Since low levels (1-2 ng/ml) of TGF-β in the serum-containing culture medium were suspected to inhibit the erythroid differentiation of WT TF-1 through its binding to CD109, WT TF-1 cells were cultured in a serum-free medium Expi293 Expression Medium for 10 days. While control TF-1 cells cultured in the serum-containing RPMI1640 were negative for the expression of GPA, 77.0-84.5% of the cultured TF-1 cells expressed GPA and exhibited erythroid morphology. CD109 was expressed by 12.1-18.3% of CD34+CD38- cells, 4.5-7.4% of common myeloid progenitor cells (CMPs), 20.8-42.4% of megakaryocyte-erythrocyte progenitor cells (MEPs), and 14.2-22.0% of granulocyte macrophage progenitor cells (GMPs) in the bone marrow of healthy individuals, while murine CD48-CD150+CD34- LSK cells were negative for either CD109 protein or mRNA. [Conclusions] CD109 protects TF-1 cells from differentiating into erythroid cells in serum-containing culture. In contrast to keratinocytes, the CD109 on TF-1 cells, and possibly on HSPCs, may enhance TGF-β signaling, and the lack of the GPI-AP might make PIGA-mutated HSPCs insensitive to TGF-β, leading to the preferential commitment of mutant HSPCs to mature blood cells in immune-mediated bone marrow failure.
Nakao:Kyowa Hakko Kirin Co., Ltd.: Honoraria; Novartis: Honoraria; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal